By CP
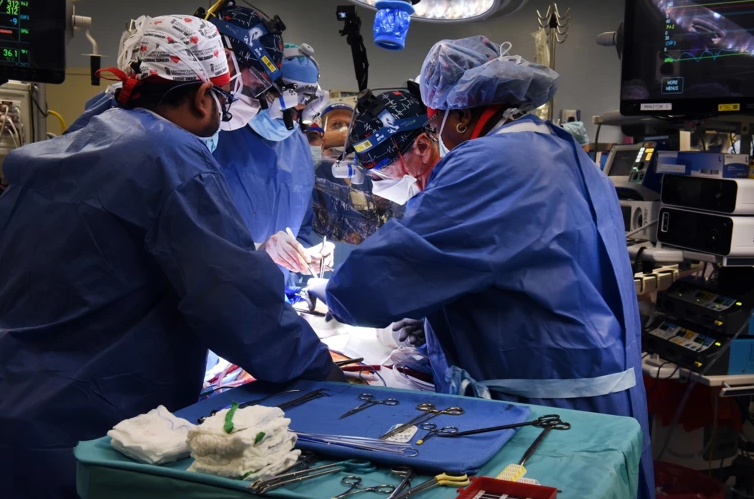
Faculty members at the University of Maryland School of Medicine in Baltimore announced Monday that they successfully transplanted a genetically-modified pig heart into a human in a “first-in-the-world surgery,” giving hope as thousands are stuck on a transplant waiting list annually and die in the process.
“This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis. There are simply not enough donor human hearts available to meet the long list of potential recipients,” Dr. Bartley P. Griffith, who surgically transplanted the pig heart into 57-year-old David Bennett, said in a statement.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” Griffith added.
Doctors said Bennett, who was suffering from terminal heart disease, was doing well three days after the procedure.
The 57-year-old father, who had been hospitalized and bedridden for the past few months, was deemed ineligible for a conventional heart transplant at UMMC, as well as several other leading transplant centers that reviewed his medical records. He explained that getting the genetically modified pig heart was his “last choice.”
“It was either die or do this transplant. I want to live. I know it’s a shot in the dark, but it’s my last choice,” he explained a day before the surgery.
The doctors were granted emergency authorization from the U.S. Food and Drug Administration for the surgery on New Year’s Eve.
According to the federal government’s organdonor.gov, about 106,000 Americans are waiting for an organ transplant, and more than 6,000 patients die each year before getting one. Last year, about 3,817 Americans received human donor hearts, but the demand remains higher, The New York Times reports.
Transplanting animal organs, known as xenotransplantation, was first attempted in the 1980s but was abandoned after a well-known case of Stephanie Fae Beauclair, whom many refer to as Baby Fae, at Loma Linda University in California.
Baby Fae was born with a fatal heart condition and received a baboon heart transplant but died within a month of the procedure due to the immune system’s rejection of the foreign heart, UMMC explained.
However, pig heart valves have been successfully used to replace valves in humans for years now.
Before consenting to the surgery, Bennett suffered from life-threatening arrhythmia and was informed of the risks.
Griffith told The New York Times that he first approached Bennett with the experimental treatment in mid-December, in a “memorable” and “pretty strange” conversation.
“I said, ‘We can’t give you a human heart; you don’t qualify. But maybe we can use one from an animal, a pig,” Griffith said. “It’s never been done before, but we think we can do it.’”
“I wasn’t sure he was understanding me,” Griffith continued. “Then he said, ‘Well, will I oink?’”
Dr. Muhammad M. Mohiuddin, a professor of surgery at UMSOM, who is also considered one of the world’s foremost experts in xenotransplantation, said in a statement that the surgery followed years of complex research.
“This is the culmination of years of highly complicated research to hone this technique in animals with survival times that have reached beyond nine months. The FDA used our data and data on the experimental pig to authorize the transplant in an end-stage heart disease patient who had no other treatment options,” he said. “The successful procedure provided valuable information to help the medical community improve this potentially life-saving method in future patients.”
Revivicor, a regenerative medicine company based in Blacksburg, Virginia, provided the genetically-modified pig for the surgery.
A total of 10 “unique gene edits” we made in the donor pig.
“Three genes—responsible for rapid antibody-mediated rejection of pig organs by humans—were ‘knocked out’ in the donor pig,” the statement from the medical center details. “Six human genes responsible for immune acceptance of the pig heart were inserted into the genome. Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue.”